Beautiful Info About How To Tell If A Molecule Is Polar Or Non

Learn to determine if a molecule is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).we start with the polarity betwe.
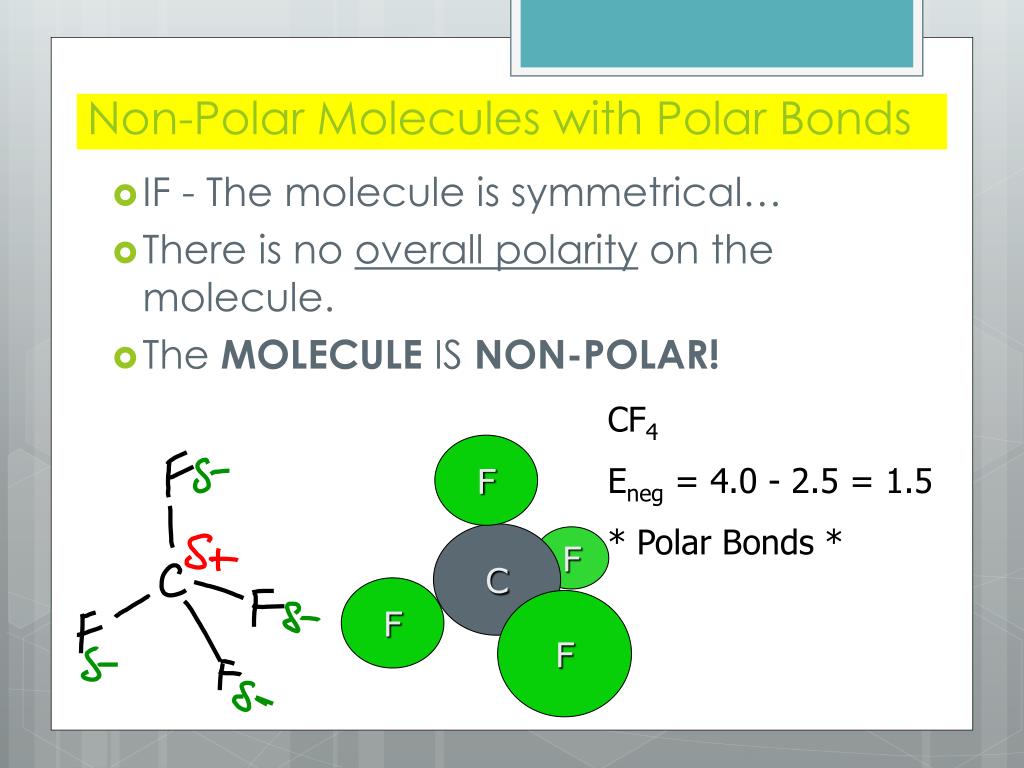
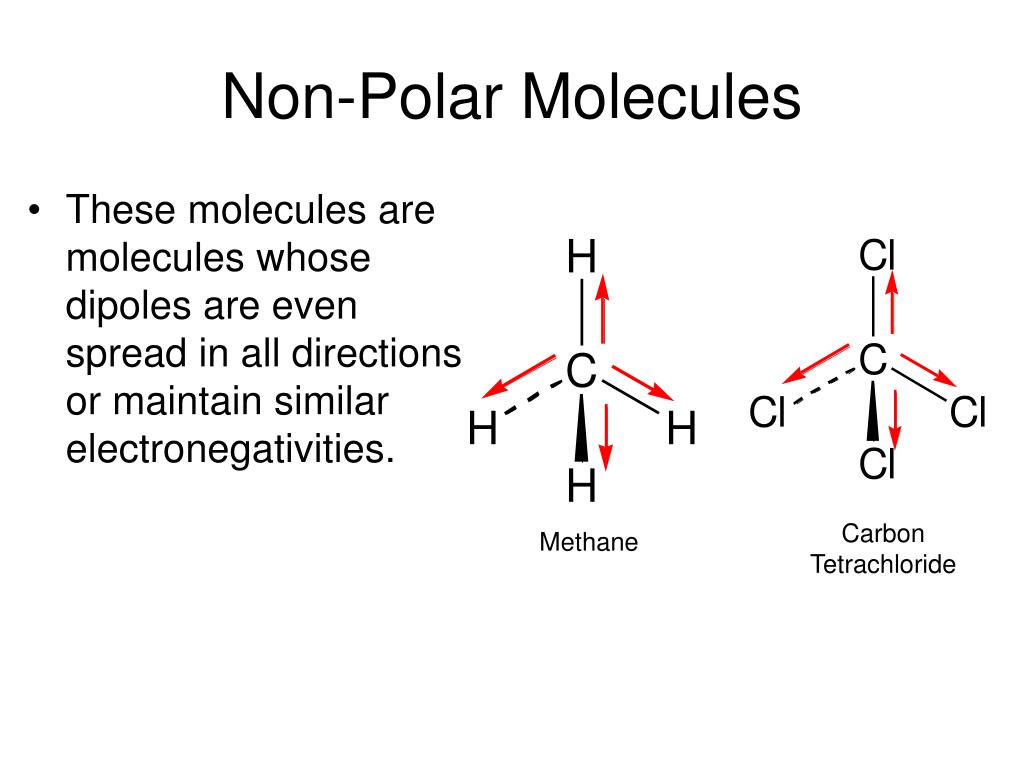
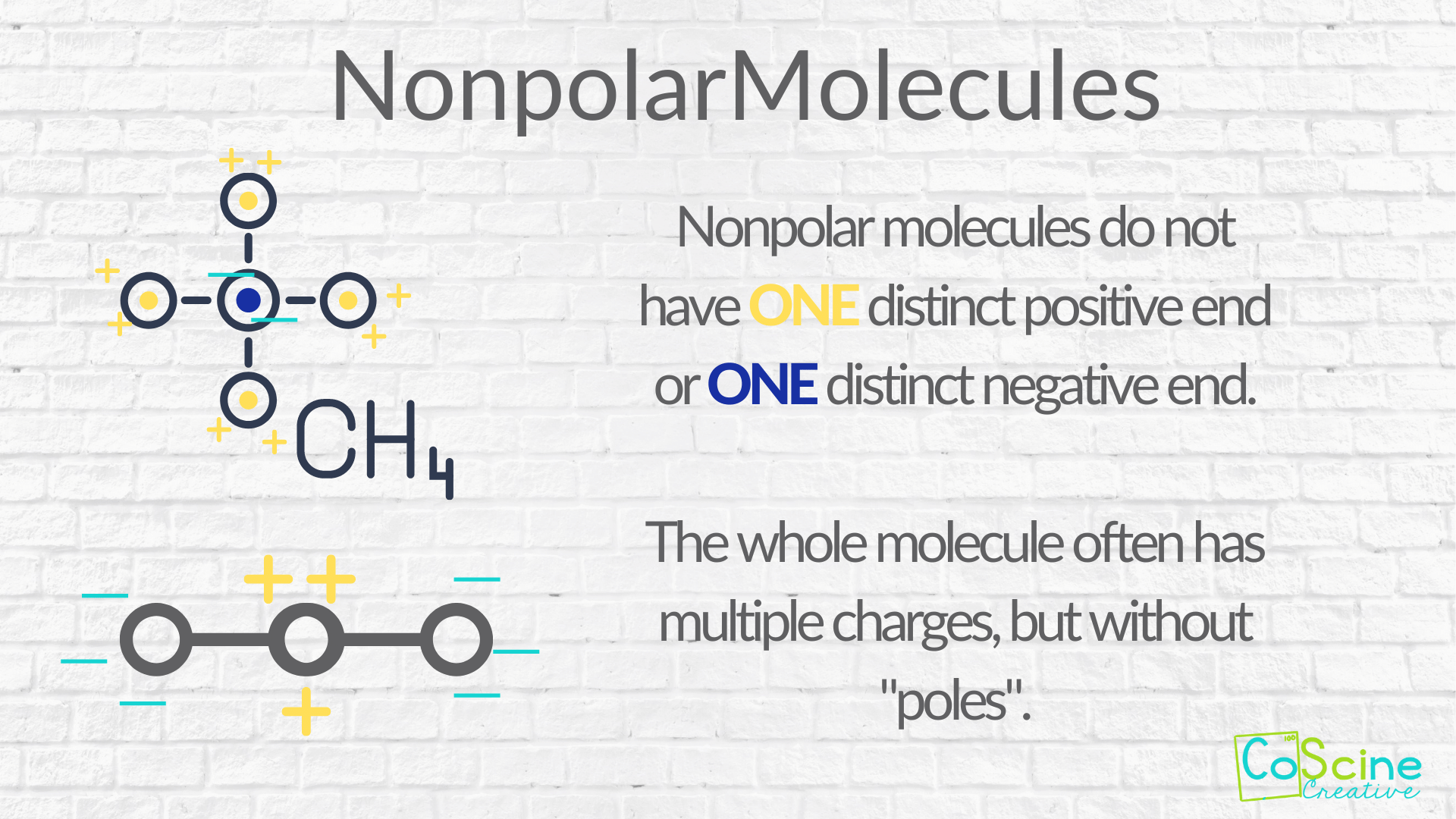
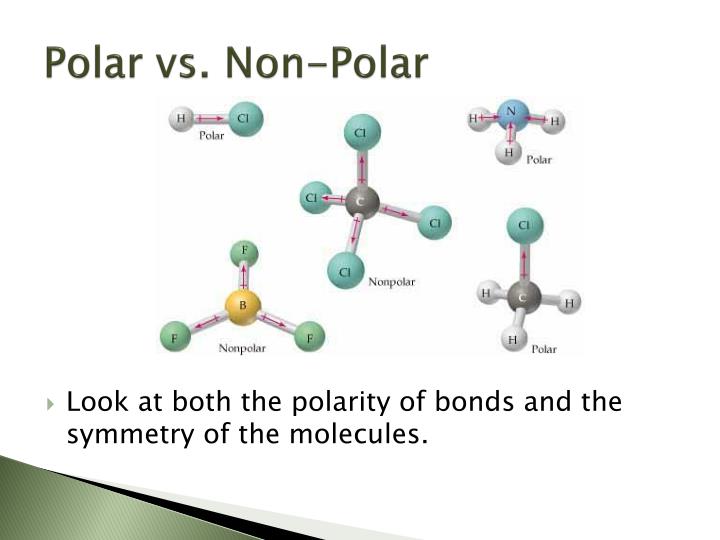
How to tell if a molecule is polar or non polar. When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. Have higher melting points than nonpolar molecules; To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures.
Therefore, they are electrically charged. Thoughts do not have mass. Polar molecules interact with other polar substances.
Na+, k+ ) these ions already exist in the neuron, so the correct. However, the following properties are typical of such molecules. Polar molecules are formed either as a result of electronegative atoms or due to asymmetric arrangement of nonpolar bonds and lone pairs of electrons on the.
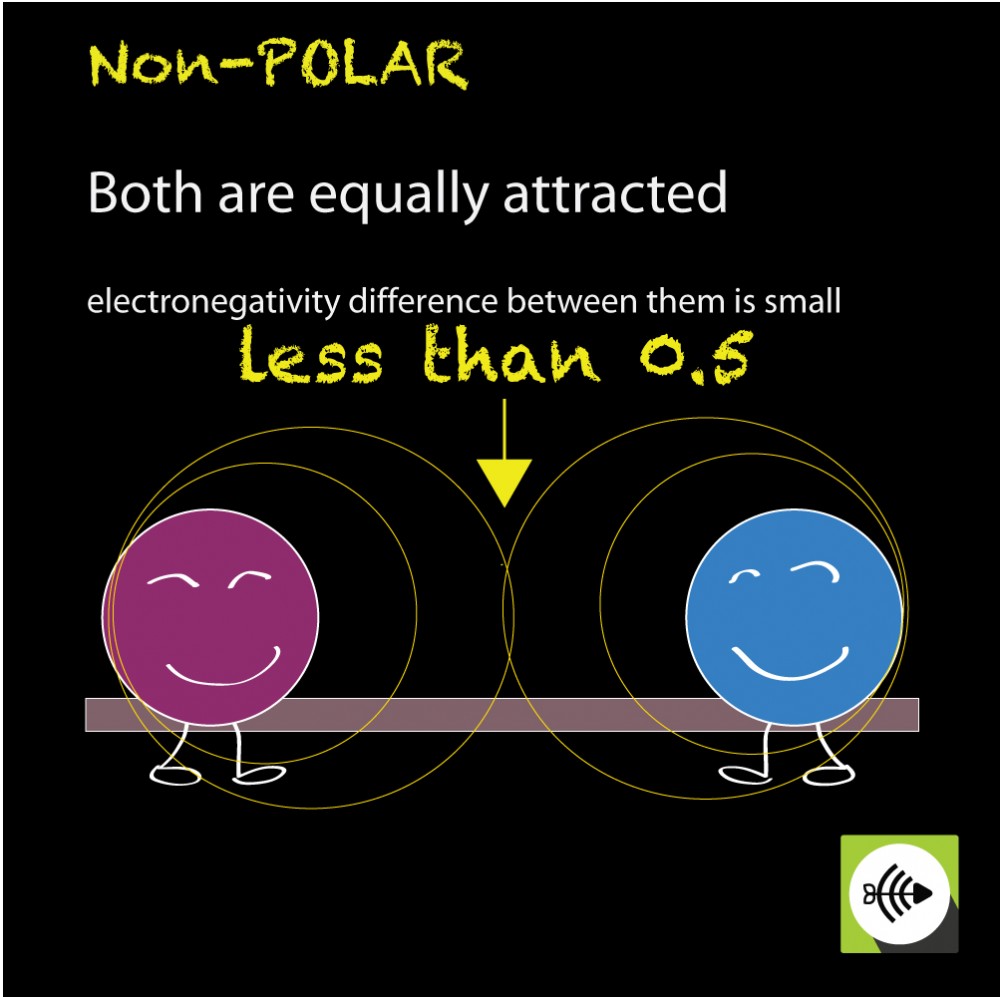
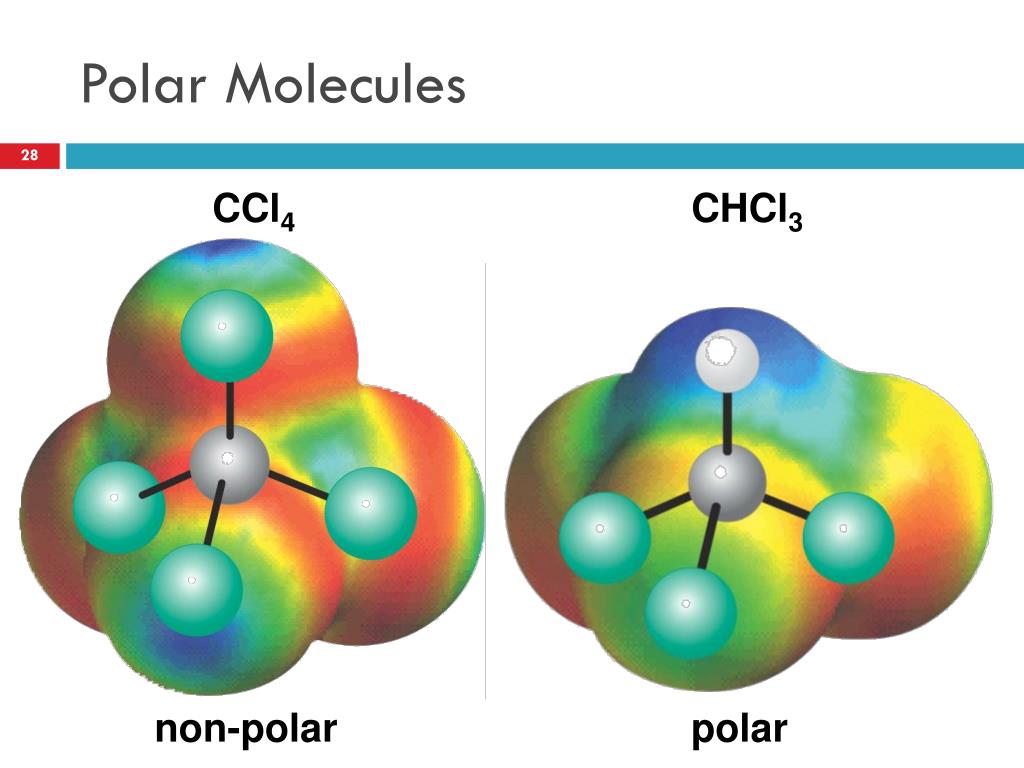
Nonpolar compounds will be symmetric,. When in a bond, atoms can either share. It provides examples so you can quickly distinguish nonpolar molecul.
The reason is because a thought merely triggers a response of ionic movement (i.e. Updated on september 02, 2020. This video provides a fast way for you to determine if a molecule is polar or nonpolar.
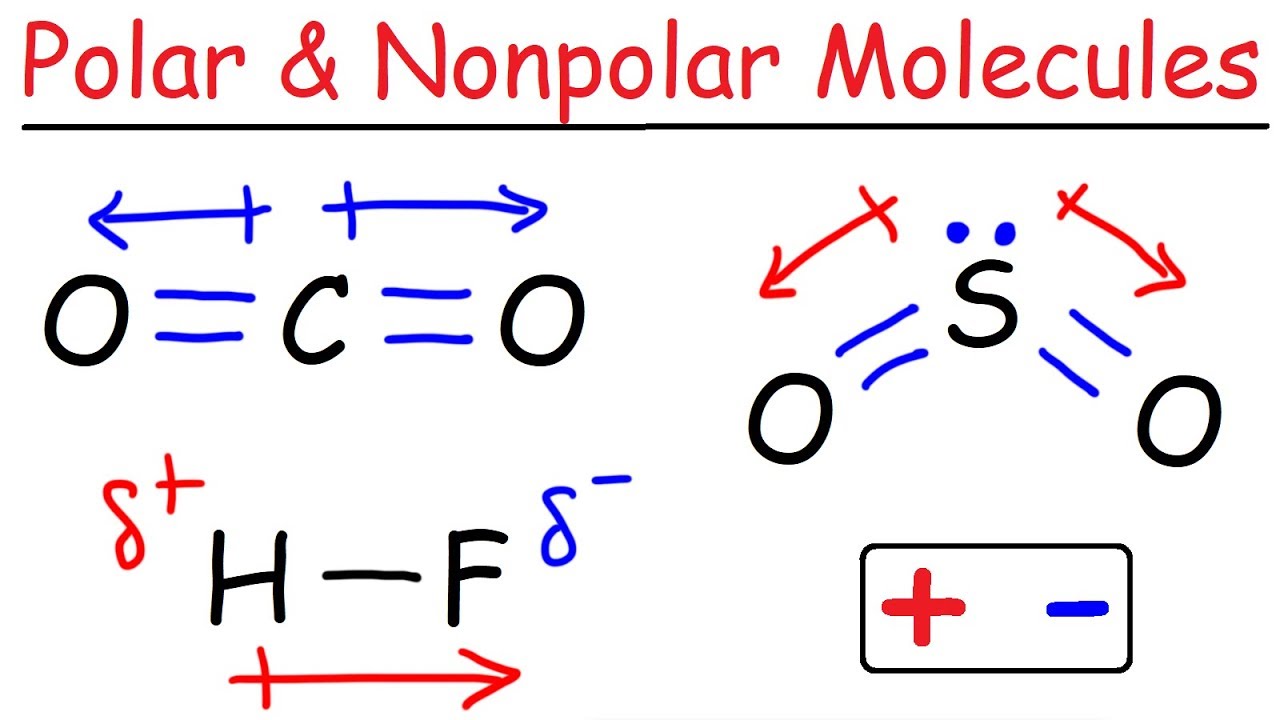
To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. These molecules have positive and negative charges on opposite ends. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.
4m views 10 years ago chemistry.





:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)